Arena Pharmaceuticals, neighbors of ours here in San Diego, developed Lorcaserin proposed as a new treatment for obesity. The compound acts as a serotonin receptor agonist subtype (5-HT 2C) triggering a cascade that promotes satiety. An FDA advisory committee voted against this proposed weight-loss pill amid concerns that tumors in rats might indicate a risk of cancer in humans, thus potential risks for long-term use and outweighed possible benefits in weight loss. Granted those benefits were small but still statistically significant.
The outcome presents many questions. On one hand the proponents of the idea that the simple a chemical the more likely to become a drug should be disappointed but it may reinforce the idea that we need “moderately complex” structures.
Chemistry aside, the way in which the FDA advisory group voted raises several questions. Of course the therapeutic area counts in voting decisions and we still not perceive obesity as a serious enough threat in the way we would consider and antineoplastic agent. Therefore the bar for passage becomes higher. The second point is that when in doubt vote against. Inaction is seldom punished. If you vote against, we will never know the opportunity lost, but if you vote yes and it does not work or causes trouble you will be stigmatized or worse. The mentality is similar to the big pharma deal making, nobody is punished for not making a deal, but if you make a deal, you own it. I am not sure the mentality is in the FDA advisory committees but it is human nature.
The third point comes to the issue of the role of the FDA. With lorcaserin there are questions about safety, which should be resolved but only to the extent it is needed. There were comments made by the panelists and captured in the press about its efficacy. The compound is effective even if it is marginally effective. If it is not snake oil and it were found to be sufficiently safe, should not the decision on the best drug to use be left to the physician and the patient? Once the compounds meet that efficacy burden (which is not trivial) the issue of efficacy should be removed from the table. For some patients a bit of help may be all they need and the risk profile of the drug may be one that the patient and doctor are willing to take (an obese 70 years old may be less concerned with potential carcinogenicity compared to the immediate risk of a diabetic complication or cardiovascular event). Safe and effective is a good standard, safe and ultra effective may leave doctors and patients with options that could be useful for some patient populations. There are also several indirect issues affected by this way of thinking. Fewer substitute or competitive products play into the issue of drug pricing, which we are all concerned about. Knowing that the standards are rational will certainly foster transparency; with companies more willing to disclose even smaller issues if they’re assured that they will not be penalized for it. A more logical standard for efficacy would be a step forward. Can this drug be useful and safe to some patients when prescribed by a fully aware physician? In the case of this particular drug, my guess is that it could have been.
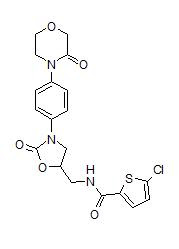
 A series of V3 antagonists were disclosed in patent 7807686 by Organon, which after a couple of mergers (what's new) is now part of the Merck/ Merck Sharp Dohme organization. The compounds share a common scaffold as shown on the left.
A series of V3 antagonists were disclosed in patent 7807686 by Organon, which after a couple of mergers (what's new) is now part of the Merck/ Merck Sharp Dohme organization. The compounds share a common scaffold as shown on the left.